Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.

Note: this_feature_currently_requires_accessing_site_using_safari
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Why does water expand when cooled below 4 degrees?
- Thread starter ríomhaire
- Start date
ktimekiller
Companion Cube
- Joined
- Oct 6, 2003
- Messages
- 4,838
- Reaction score
- 40
no idea
Puzzlemaker
Newbie
- Joined
- Aug 1, 2004
- Messages
- 914
- Reaction score
- 0
I know why.
Let me explain.
When water turns to ice, it begins to crystilize. In its liquid form, all the water molocules are bouncing around, close together and quite happy. When it begins to crystilize, hydrogen bonds behind to take place.
Think of it as the diffrence between having a pile of sticks and making something out of them, lets say a fort. The fort you make would be stronger and take up more room then the pile of sticks.
At four degress it starts to crystilize, and expand. If you get it cooler it solidifies.
Let me explain.
When water turns to ice, it begins to crystilize. In its liquid form, all the water molocules are bouncing around, close together and quite happy. When it begins to crystilize, hydrogen bonds behind to take place.
Think of it as the diffrence between having a pile of sticks and making something out of them, lets say a fort. The fort you make would be stronger and take up more room then the pile of sticks.
At four degress it starts to crystilize, and expand. If you get it cooler it solidifies.
Ravioli
Microboner
- Joined
- Nov 1, 2004
- Messages
- 5,095
- Reaction score
- 2
you mean 0? Or are you using Farenheith? It change its state from liquid to solid. The atmos are arranged in a different way:
Gas-The atoms are not packed, they are completely loose
Liquid- The atoms are more packed but still a bit loose
Solid (ice)- Atoms are completely packet together
However, i have no idea how that relevates to this discussion so i will stfu right now.
EDIT: Maybe it was molecules, not atoms
Gas-The atoms are not packed, they are completely loose
Liquid- The atoms are more packed but still a bit loose
Solid (ice)- Atoms are completely packet together
However, i have no idea how that relevates to this discussion so i will stfu right now.
EDIT: Maybe it was molecules, not atoms
- Joined
- Aug 8, 2004
- Messages
- 12,231
- Reaction score
- 241
Precisely, it forms a crystaline structure with spaces between molecules of water with hydrogen bonds between the hydrogen and oxygen atoms. They are also held there in that shape because of those bonds, and so the ice is a solid.
Cole
Newbie
- Joined
- Jan 19, 2004
- Messages
- 6,427
- Reaction score
- 1
Almost, because if ice's atoms were more tightly packed then it would be smaller and more dense. Thats not the case.you mean 0? Or are you using Farenheith? It change its state from liquid to solid. The atmos are arranged in a different way:
Gas-The atoms are not packed, they are completely loose
Liquid- The atoms are more packed but still a bit loose
Solid (ice)- Atoms are completely packet together
However, i have no idea how that relevates to this discussion so i will stfu right now.
EDIT: Maybe it was molecules, not atoms
Take just about any liquid besides water, cool it into a solid. Drop the solid into its liquid form. It will not float because it is more dense, the atoms are more tightly packed.
Now take water, cool it to ice. Drop the ice into water. It will float. As Glenn said, The atoms form a crystaline structure and take up more space, more space = less dense. This allows ice to float on water.
marksmanHL2 :)
Spy
- Joined
- May 29, 2003
- Messages
- 5,192
- Reaction score
- 0
Oh you clever monkey's! 
I learn something knew every day here.
Admitadly most of its meaningless rubish... but meh!
I learn something knew every day here.
Admitadly most of its meaningless rubish... but meh!
- Joined
- Aug 8, 2004
- Messages
- 12,231
- Reaction score
- 241
How very dare you Mark!?Admitadly most of its meaningless rubish... but meh!
Beerdude26
Party Escort Bot
- Joined
- Oct 20, 2004
- Messages
- 10,350
- Reaction score
- 1
Hydrogen bonds methinks. I don't think it occurs with any other compound except the ones the have water in them.Why does this not happen with most other compounds and what others does it happen in?
theotherguy
Newbie
- Joined
- Jul 5, 2003
- Messages
- 5,107
- Reaction score
- 1
Let me explain,when water is a liquid at higher temperatures, it is tightly packed together and it slides around because the hydrogen bonds are in small angles, like this

When water freezes, the molecules line up, and the hydrogen bonds become more rigid it basically looks like this
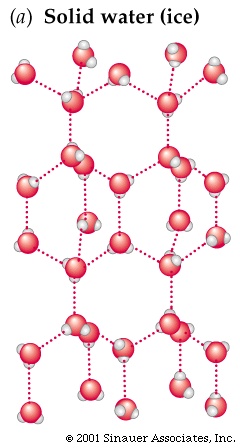
as you can see, the bonds spread the molecules out and thus it expands

When water freezes, the molecules line up, and the hydrogen bonds become more rigid it basically looks like this

as you can see, the bonds spread the molecules out and thus it expands
Puzzlemaker
Newbie
- Joined
- Aug 1, 2004
- Messages
- 914
- Reaction score
- 0
Ya, water is a very strange substance. Also, because of the polarity of the water molocule, it can disolve stuff, like salt.
Simpler version: Water has a plus and minus side, like a magnet. This lets stuff stick to it, AKA disolve into the water.
You can see in the picture the other guy put up that the hydrogen, which has a... negative (Maybe) charge is attracted to the oxygen atom, which has a positive (Maybe) charge. This is what causes the ice.
Science is fun!
Simpler version: Water has a plus and minus side, like a magnet. This lets stuff stick to it, AKA disolve into the water.
You can see in the picture the other guy put up that the hydrogen, which has a... negative (Maybe) charge is attracted to the oxygen atom, which has a positive (Maybe) charge. This is what causes the ice.
Science is fun!
DrDevin
Tank
- Joined
- Oct 19, 2004
- Messages
- 1,044
- Reaction score
- 0
Generally, water expands when it freezes because of its molecular structure, in tandem with the unusual elasticity of the hydrogen bond and the particular lowest energy hexagonal crystal conformation that it adopts under standard conditions. That is, when water cools, it tries to stack in a crystalline lattice configuration that stretches the rotational and vibrational components of the bond, so that the effect is that each molecule of water is pushed further from each of its neighboring molecules. This effectively reduces the density ρ of water when ice is formed under standard conditions.
Why type it out when you can just copy/paste from wikipedia?
- Joined
- Dec 31, 2004
- Messages
- 20,872
- Reaction score
- 435
It expands because they are mostly water.What if you freeze coke or blood?
Similar threads
- Replies
- 27
- Views
- 93K